Introduction to Cooling Water System Fundamentals
Cooling of process fluids, reaction vessels, turbine exhaust steam, and other applications is a critical operation at thousands of industrial facilities around the globe, such as general manufacturing plants or mining and minerals plants. Cooling systems require protection from corrosion, scaling, and microbiological fouling to maximize performance, preserve equipment life and reliability, and most importantly, help ensure employee safety.
In this chapter, we examine fundamental cooling system design and heat transfer basics. This overview will provide the foundation for the cooling water treatment discussion in the next chapter.
Interested readers are also encouraged to explore the Cooling Technology Institute’s website. This long-standing organization provides excellent information on all facets of industrial and commercial cooling applications.
Table of Contents
Types of Cooling Systems
Cooling Tower Components
Overview of Natural Draft Cooling Towers
Cooling Tower Heat Transfer
Sidestream Filtration
Note About Tower Performance Monitoring
Cooling Tower Alternatives
Closed Loop Water Cooling
Heat Exchangers
Heat Transfer Fundamentals
Heat Exchanger Performance Monitoring
Conclusion
Types of Cooling Systems
The three major cooling system designs are once-through, open recirculating (cooling tower-based), and closed. The first two typically serve as primary cooling for the largest heat exchangers, with closed loops for auxiliary plant systems. The fundamentals of each are outlined below.
Once-Through Cooling Systems
As the name “once-through” implies, the cooling water comes from an external source such as a lake, river, or even the ocean. After serving the heat exchangers, the water is directly discharged back to the original source. A common example, especially in the last century, was turbine exhaust steam cooling at large power plants, as shown below.
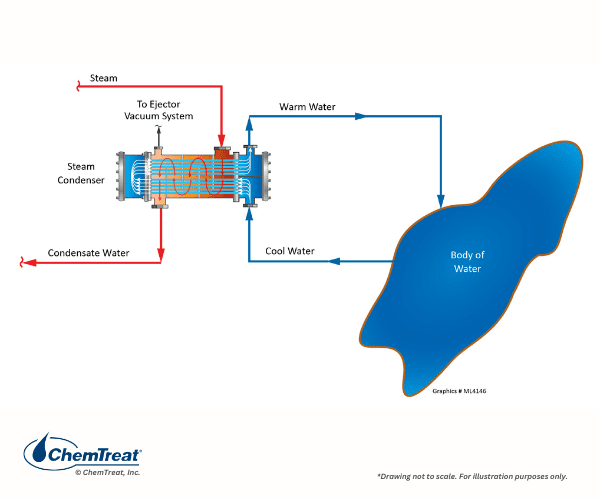
Once-through intakes are normally equipped with bar screens and/or traveling screens to remove material such as tree branches, leaves, and other large items, including aquatic life, that would otherwise physically foul condenser and heat exchanger tubes. Years ago, it became evident that the screening process was fatal to many aquatic organisms, which either violently impinged on or became trapped against the screens. Increased concern about protecting aquatic life has brought about change to cooling system design and selection with a stronger focus on sustainable water solutions and advancements in sustainable water cooling. Some existing intakes have been retrofitted with modern screens that minimize harm to aquatic life, while for many modern plants once-through cooling is no longer permitted, rather cooling tower systems are required.
Note: While many nuclear plants have cooling towers, once-through backup systems are common for emergency cooling.
Also of concern with once-through systems is the discharge of warm cooling water to the supply source. Warm temperatures can be lethal to some organisms, while others such as fish will congregate at the discharge during cold weather months. Some plants were designed with discharge channels to allow the water to cool somewhat before entering the primary water body.
In a few once-through applications, a spray system assists with discharge cooling. Similar to the cooling tower process, which is examined in detail next, a spray system enhances cooling by evaporation of a small portion of the discharge.

“Grey Water Pond at Palo Verde” by NRCgov is licensed under CC BY 2.0.
Chemical treatment of once-through systems is often straightforward but still very important to minimize micro- and macro-biological fouling and scale formation. These topics are covered in Chapter 7.
We will now examine alternatives to once-through cooling.
Recirculating Cooling Systems
In recirculating cooling systems, the water is recycled continuously. The simplest form of a recirculating cooling system is a cooling pond. Most cooling is by sensible heat transfer with minor evaporative heat loss that increases on windy and warm days. Cooling ponds require a large footprint, and thus open-recirculating systems are much more common.
Open Recirculating Cooling Systems
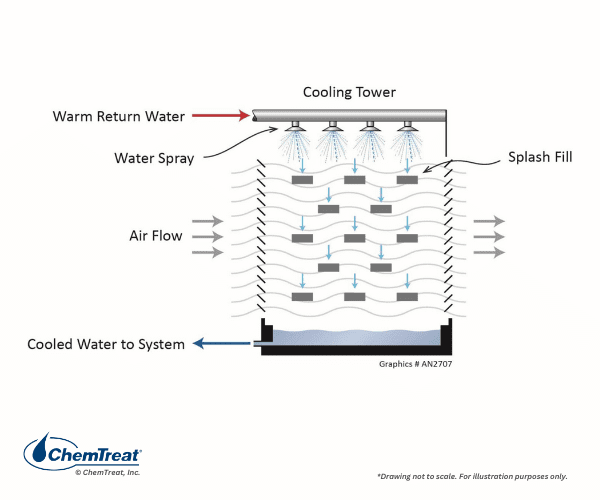
The ability to transfer large quantities of heat via a small amount of recirculating water evaporation is the basis behind cooling tower applications.

The fundamental process is shown below:
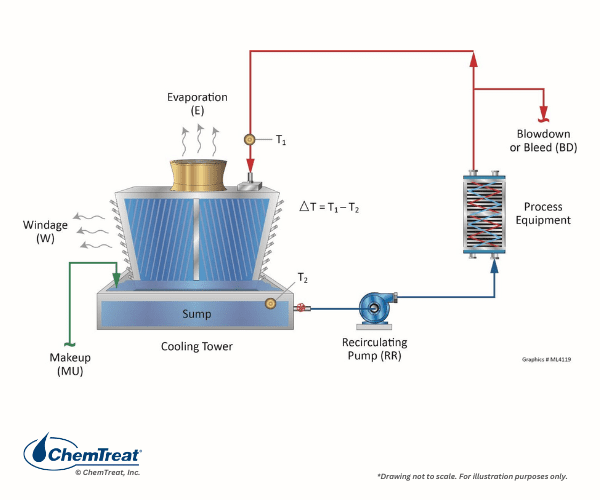
Millions of cooling towers are in service around the globe at facilities ranging in size from huge industrial plants to commercial facilities such as office buildings.
Modern cooling towers are of two primary types, mechanical draft (fans move air through the tower) and natural draft (air flows naturally through the tower.) The latter is the huge hyperbolic towers at large coal or nuclear power plants, and are much less common than mechanical-draft towers, which are the primary focus of this section.
An advantage of mechanical-draft towers is that they can be designed and assembled in cells that sit side-by-side within a common structure. Individual cells may be placed into or taken out of service to handle changing loads. The towers may be either forced-draft, in which the fans push air through the tower, or induced-draft where the fans pull the air through.

Most large industrial towers are induced-draft, but smaller units are often forced-draft to simplify operation. In forced-draft towers the air velocity decreases during air passage through the tower. The lower velocity can lead to recirculation of exhaust air to the tower inlet, reducing efficiency.
Another primary differentiation is crossflow or counter-flow, in which the air flows perpendicularly or counter-currently, respectively, to the water flow path.
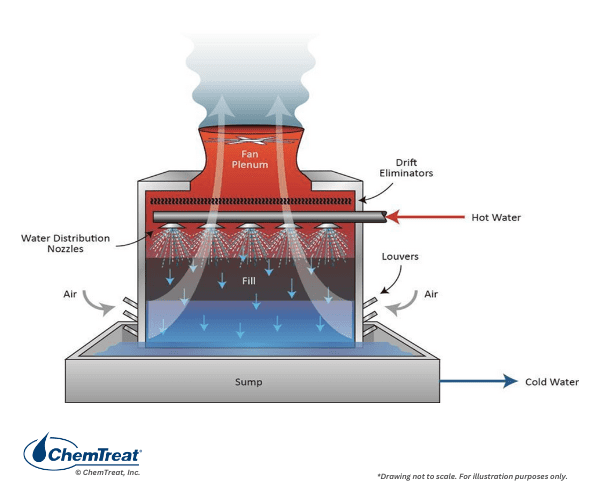
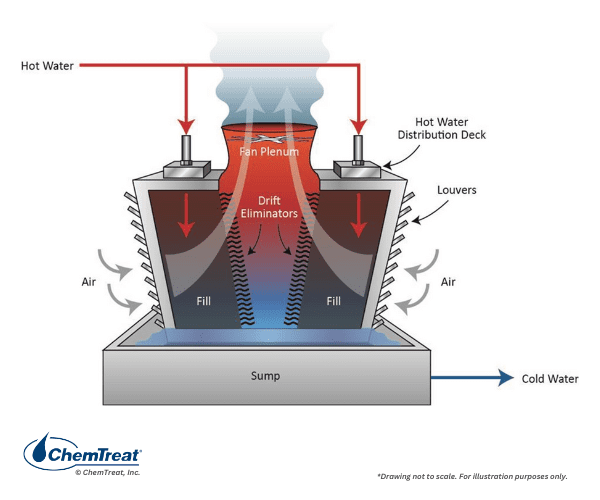
Note that the towers shown in both Figures 6.6 a and b are dual-entry types, in which the air enters from opposite sides. These are more efficient than single-entry towers, where wind direction has a greater impact on efficiency. Large towers are often placed to take advantage of prevailing wind patterns. Occasionally, one might see an octagonal or circular tower for maximum efficiency regardless of wind direction, but the design and construction costs of these towers are greater than for standard rectangular towers, and thus they are not that common.
Cooling Tower Components
The tower components shown in the previous illustrations are critical for successful structural stability and operation. The next sections review the most important of these.
Structural Materials
Depending on size, age, and other factors, the structural supports and internal components of cooling towers may be of several materials. Knowledge of the various materials for any application is important for optimizing water treatment, as is discussed in Chapter 7.
In past years, large cooling towers had wooden support structures. Pressure-treated Douglas Fir and Redwood were the two most common choices. The primary advantages of these materials are reasonable cost, decent strength, and that the products can be easily cut to precise specifications in the field. Disadvantages include:
- Susceptible to fungal decay in the cooling tower plenum
- Susceptible to chemical attack in wetted zones
- Chlorine
- Low or high pH
- Combustible (destruction by fire of cooling towers is very well known)
- Variable degrees of wood quality, either from the source or, more commonly, the steps taken in the pressure treatment process.
The replacement for wood in many modern, large industrial towers is fiberglass-reinforced plastic (FRP).

Benefits of fiberglass include:
- Decay resistant, especially compared to wood
- No chemical leaching
- Reduced flammability
- Chemical resistance is generally outstanding. FRP can tolerate high concentrations of chlorides and sulfates, which promote corrosion in cooling towers constructed of metals.
- Soft water is not aggressive to FRP
Limitations of fiberglass include:
- High temperatures can be problematic.
- Field repairs are virtually impossible. Once a section is damaged mechanically, it usually must be replaced.
- General lack of stiffness, relative to steel and wood, which results in limitations for use where mechanical loading might be large.
Typical for large cooling towers, and as is shown in Figure 6.8, is a concrete water basin. This can at times present corrosion challenges, as will be discussed in Chapter 7. In such cases, advanced methods can be used to protect cooling water systems as well as cooling water chemistry programs.
For smaller cooling towers such as those at commercial buildings, galvanized steel is a common structural material. Small towers can often be fabricated on one skid in the supplier’s shop and shipped to the site directly.

Galvanized towers may have a carbon steel basin. Other small towers may be fabricated from stainless steel, sometimes in the mistaken belief that stainless is resistant to all forms of corrosion.
Tower Fill
The primary method of heat transfer in a cooling tower is evaporation of a small portion of the recirculating water. Key to maximum heat transfer (within various water quality restraints as we shall see) is correct fill selection. Proper selection lowers the liquid-to-gas (L/G) ratio for the tower, and correspondingly reduces the size and material/operational costs of the tower and of auxiliary equipment such as recirculating pumps and fans.
Early cooling towers had wooden splash fill; a series of staggered slats below the water spray or distribution nozzles.
Water impinging on the slats breaks into small droplets that increase the surface area.
Splash fill is common for crossflow towers, and the technology has been considerably improved, with a modern design shown below.

Splash fill may be the only choice in cooling towers where the water has a high fouling tendency, but in most towers film fill is the preferred material, as it enhances air-water contact. Typical film fills are made of PVC per low cost, durability, good wetting characteristics, and inherently low flame spread rate. Film fill is not generic in nature, and numerous designs are available. The choice of flow configuration and the spacing between the fill sheets (flute size) must be evaluated carefully, and are dependent upon the projected quality of the recirculating water. The following illustrations outline several film fill styles ranging from a low-fouling design for waters with strong fouling potential to high-efficiency types.
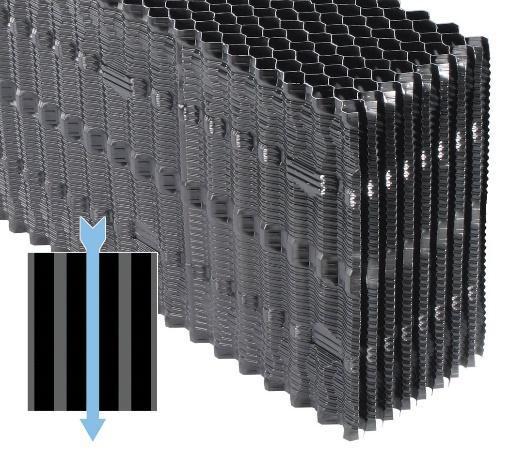

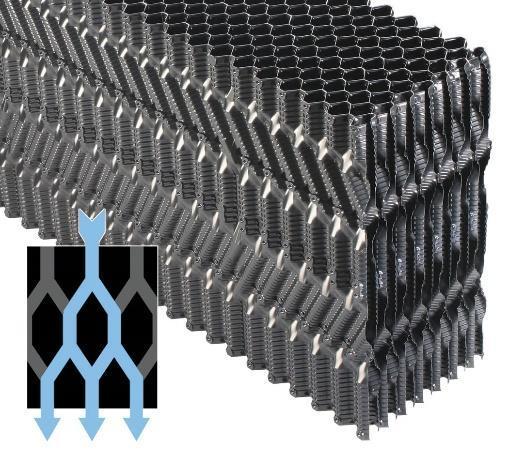

Figures 6.11a–d show a progression of various film fill configurations moving from low efficiency and corresponding low fouling potential to high efficiency and high fouling potential.
Cooling tower manufacturers continue to improve efficiency, but this is a double-edged sword in that the complex flow path increases potential locations for solids deposition. The following table outlines general guidelines for some of the designs shown above.
Table 6-1. Fill Selection Based on Water Quality1
Source: Reference 2
| 19 mm CF | 21 mm OF | 19 mm VF | 25 mm M/S | 38 mm VF | 19 mm XF- Standoff4 | |
| Allowed TSS (ppm) with Good Microbial Control2 | <100 | <200 | <500 | <1,000 | No Limit | <500 |
| Allowed TSS (ppm) With Poor Microbial Control3 | <25 | <50 | <200 | <500 | <1,000 | <200 |
| Allowed Oil and Grease (ppm) | None | <1 | <5 | <50 | <25 | <5 |
| Fibers | None | None | None | None | None | None |
- These are general guidelines and may need modification based on site-specific conditions.
- ‘Good’ microbiological control means oxidizing biocide supplied continuously with free oxidant residuals maintained, with total aerobic bacteria (TAB) maximum plate counts not exceeding 100,000 cfu/ml (colony forming units) with minimal slime formation on heat transfer surfaces.
- ‘Poor’ microbiological control implies little or no microbiological control or control subject to severe disruption, with average TAB plate counts consistently over 100,000 cfu/ml. Other potential fouling risk factors must be considered also, such as water-borne cross-contamination with process fluids containing ammonia compounds, sugars or other nutrients. Other airborne contaminants should be considered also, such as fine dust, dirt, and debris.
- For high water loading typically found in crossflow towers
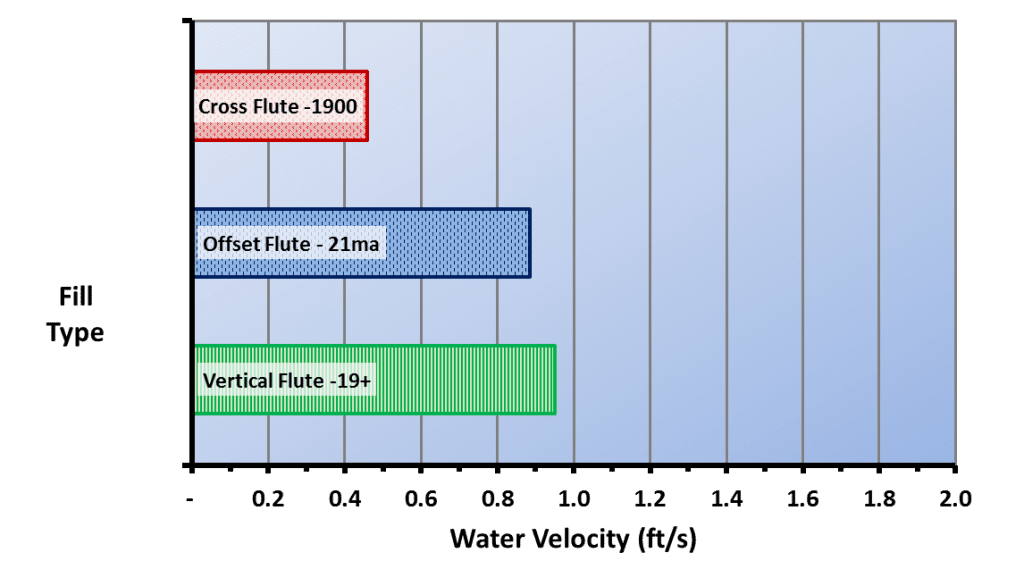
Figure 6.12 illustrates the effect of water velocity on the depth of biofilms.
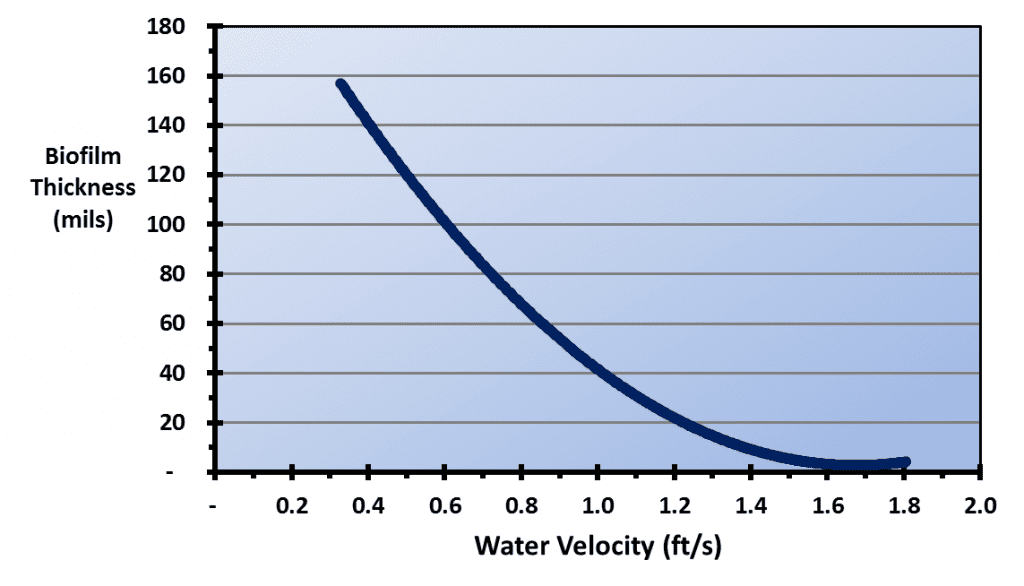
A comparison of this illustration with the types of cooling tower fill shown above highlights the vulnerability of cellular plastic film packs to biofouling. The water film velocity in typical cross-fluted film packs has been reported to be only 0.48 ft/s, and for fouling-resistant film packs, only 0.89 ft/s – 0.95 ft/s for an 8 gpm/ft2 water loading rate.
Biofilms collect suspended solids that enter the tower via the makeup and air flow to produce mud-like deposits that can become very thick.

The deposits can close off fill passages, which, of course, reduces air-water contact and degrades heat transfer. Deposition can also add enormous weight to the fill. Both effects are clearly shown below.
In extreme cases, fouled fill has collapsed, resulting in an unscheduled outage and large replacement costs. Fortunately, there are modern techniques for corrosion and fouling protection.

ADVANCED OXIDIZING BIOCIDE SELECTION FOR DIFFICULT COOLING AND PROCESS WATERS
Microbiological colonies tend to accumulate in the middle of the fill pack. Water velocities directly under the spray nozzles are generally high enough to discourage microbe adhesion. Additionally, fouling tends to be more intense in the middle of the fill than at the bottom because suspended solids are filtered out prior to reaching the lowest fill layer and because the last few inches of fill do not physically support a soft deposit mass. The absence of microbial colonies at either the top or bottom of the fill, combined with the difficulty of inspecting the middle layers, often allows fouling to progress undetected until it has reached an advanced stage. Personnel at power plants and industrial facilities have attempted to monitor fill fouling during tower operation using sections of fill suspended from load cells, or by cutting an access window into the end of the tower casing to allow a middle section to be removed periodically for inspection using a man lift, or by suspending a section of fill beneath the main fill pack to allow it to be easily inspected and weighed. These monitoring techniques may be somewhat effective, but none have proven to be totally satisfactory.
Several methods exist to remove biological/silt deposits from cooling tower fill. Hyper-halogenation is one method, but effectiveness may be limited. Furthermore, the high chlorine concentration can cause corrosion to system components, and, when the cleaning is complete, the waste stream may require treatment before discharge. Microbiological colonies have high water content and will shrink and detach from surfaces when thoroughly dried. US Patent 5,558,157 outlines this method for biofilm removal in shell and tube heat exchangers. However, effectively drying out cooling tower fill can prove problematic even with the help of fans. Chlorine dioxide has also served as a cleaner for cooling tower biofilms with some success.
The most widely practiced and effective cleaning chemical for microbiological deposits is hydrogen peroxide (H2O2). Peroxide is effective due to its oxidizing strength and from the physical action of oxygen micro-bubbles produced as it reacts with organic deposits. The decomposition products of peroxide are water and oxygen, and thus the compound has a very positive environmental profile. Typical dosages are in the 500-3,000 ppm range of active chemical. As with most cleaning processes of this type, addition of low levels of surfactants will help loosen deposits. Polymeric dispersants are often also included to keep dislodged solids in suspension until they can be discharged.
Mist Eliminators
The interaction of air and water in the tower generates many fine droplets that can potentially exit the tower in the plume. The common term for this loss is “drift.” Moisture discharge is problematic for two reasons. First, solids within the droplets can deposit on induced-draft fan blades and gradually impact performance. Secondly, plant air emissions regulations usually also include cooling tower discharge. A facility may be in violation of discharge guidelines from the solids entrained in the droplets. Accordingly, chevron-vane mist eliminators are standard cooling tower items. The demisters collect water by impingement and allow the water to drain back into the tower.

Technology has advanced such that modern demisters can reduce entrained moisture to less than 0.0005% of the recirculating water rate. To put that into perspective, the drift from a tower with a 100,000 gpm recirculation rate and 0.0005% drift would be 0.5 gpm. Very slight indeed!
Water Distribution System
Critical to efficient tower operation is proper design of the water distribution system. The figure below shows a portion of a modern spray system for a counter-flow tower.

Crossflow towers normally have a distribution deck above the fill, where a stable level of water is maintained on the deck surface. The water flows onto the fill via a precise pattern of deck perforations.

Fill requires uniform water distribution to minimize channeling. Channeling on its own will reduce tower efficiency, but it can also establish low-flow locations that allow increased deposition and formation of microbiological colonies. In counter-flow towers with water distribution networks, regular inspections should include examination of the spray nozzles to find plugged, broken, or missing nozzles. For crossflow towers, plugging of the distributor deck perforations by solid materials or algae can cause mal-distribution.
Normally, recirculating systems are designed with multiple pumps so that the water flow rate can be adjusted per seasonal temperature changes. Also, in typical multi-cell towers, cells can be placed in or removed from service depending upon cooling requirements.
Fans
As previously noted, most large cooling towers have induced draft fans. Important aspects of fans beyond size and motor horsepower include fan speed and blade pitch. Fan air flow can stall if the fan settings are not correctly configured. Regular monitoring and maintenance are important. Fans may become unbalanced and misaligned from the accumulation of deposits that exit with the plume. Gearboxes are another item that require regular inspection and lubricant monitoring.
A not uncommon arrangement is dual-speed fan control, or perhaps even greater flexibility with variable frequency drive (VFD) control. Thus, rather than placing a cell or cells in or out of service to adjust for load or ambient air temperature changes, the fan speed can be modified. This adjustment may be especially beneficial during the winter to minimize icing. Also in that regard, the reader might recall that some of the previous tower diagrams in this chapter showed inlet louvers. These can be adjusted to modify air flow.
A Brief Overview of Natural Draft Cooling Towers
Throughout several decades of the last century, hyperbolic cooling towers were often selected at large power plants because they could be rolled into the overall plant costs, and required no fans.
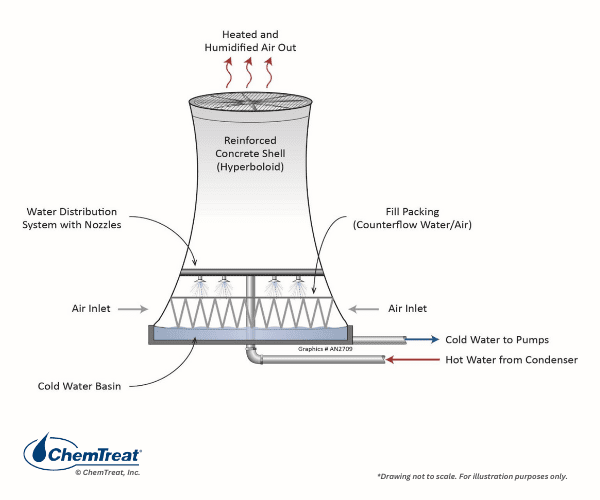
As the figure indicates, hyperbolic towers are very large, tall structures; fabricated from concrete. The design allows natural airflow (from any direction) and structural stability. The public often confuses hyperbolic towers with nuclear power plants when there is nothing “nuclear” about the towers, some of which were installed at large coal plants. Hardly any hyperbolic towers have been erected in the United States in the last several decades, and will not be discussed further in this book.
Cooling Tower Heat Transfer
As air passes through a cooling tower, it induces evaporation. The water that evaporates consumes a large amount of energy during the change in state from a liquid to a gas. This is known as the latent heat of vaporization, which at sea level is typically around 1,000 Btu/lb. So, cooling towers remove much heat from the recirculating water by a small amount of evaporation.
An important concept for understanding cooling tower heat transfer is that of “wet bulb” temperature. Consider being outdoors, but in the shade, on a 90°F day at 40% relative humidity. A standard thermometer would read 90°, which is the “dry bulb” temperature. Now, imagine if we placed another thermometer alongside the dry bulb thermometer, but in this case have wrapped a soaked piece of cloth around the bulb of the second thermometer, and have put both on a swivel such that the thermometers can be swirled very rapidly through the air. This instrument, a simple and common device, is known as a sling psychrometer.

After a short time, the dry bulb thermometer will still read 90°F but the other thermometer will read 71.2°F. This latter reading is the wet bulb temperature, and is the lowest temperature that can be achieved by evaporative cooling. Modern psychrometers are mechanically aspirated (fans move air across the wetted wick) and are even more precise.
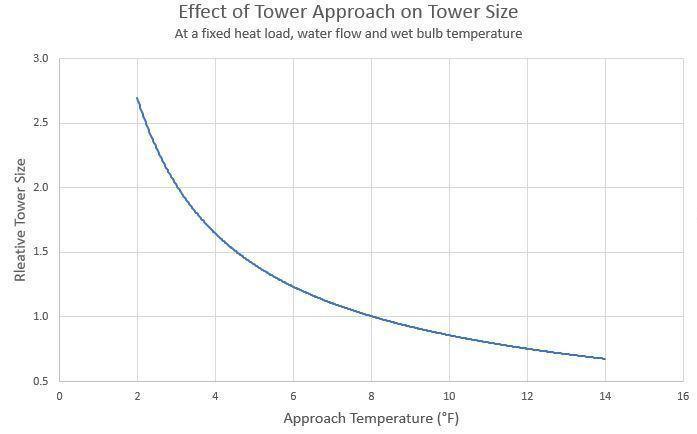
No matter how efficient, a cooling tower can never chill the recirculating water to the wet bulb temperature, and at some point, costs and space requirements limit cooling tower size. The separation in temperature between the chilled water and wet-bulb value is known as the approach.
A graphical representation of range and approach, reproduced from Reference 1, is illustrated below. Obviously, these values will be variable over the wide range of conditions in which cooling towers operate.
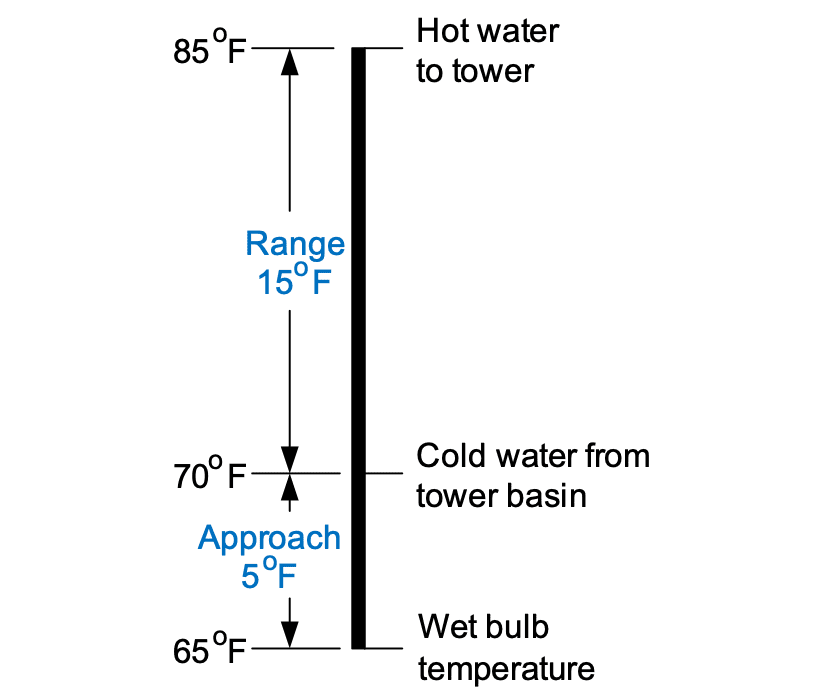
The closest approach to wet bulb temperature that can economically be reached with a modern tower is about 4°F, with a typical value being 10°F.
The data needed to calculate heat transfer by air cooling and evaporation has been compiled in a graph known as a psychrometric chart. One version is shown below.
Psychrometric charts contain a large amount of data, and can sometimes be difficult to interpret. Appendix 6-1 outlines how to evaluate this data.
Reference 8 provides a direct example of how to calculate cooling tower evaporation from psychrometric data, but a simpler equation is available that provides good approximations.
E = (ƒ * R * ΔT)/1000 | Eq. 6-1
E = Evaporation in gpm
R = Recirculation rate in gpm
ΔT = Temperature difference (range) between the warm and cooled circulating water (oF)
ƒ = A correction factor that accounts for evaporative and sensible heat transfer, where ƒ (average) is often considered to be 0.75 to 0.80, but will rise in summer and decline in winter.
The factor of 1,000 is the approximate latent heat of vaporization (Btu/lb) of water at ambient conditions.
As an illustration of this calculation, consider a cooling tower at the following conditions:
- R = 150,000 gpm
- ΔT = 15oF
- ƒ = 0.80
For these parameters, E = 1,800 gpm. Thus, the required cooling is achieved by just 1.2% evaporation of the recirculating water, with 20% sensible heat transfer.
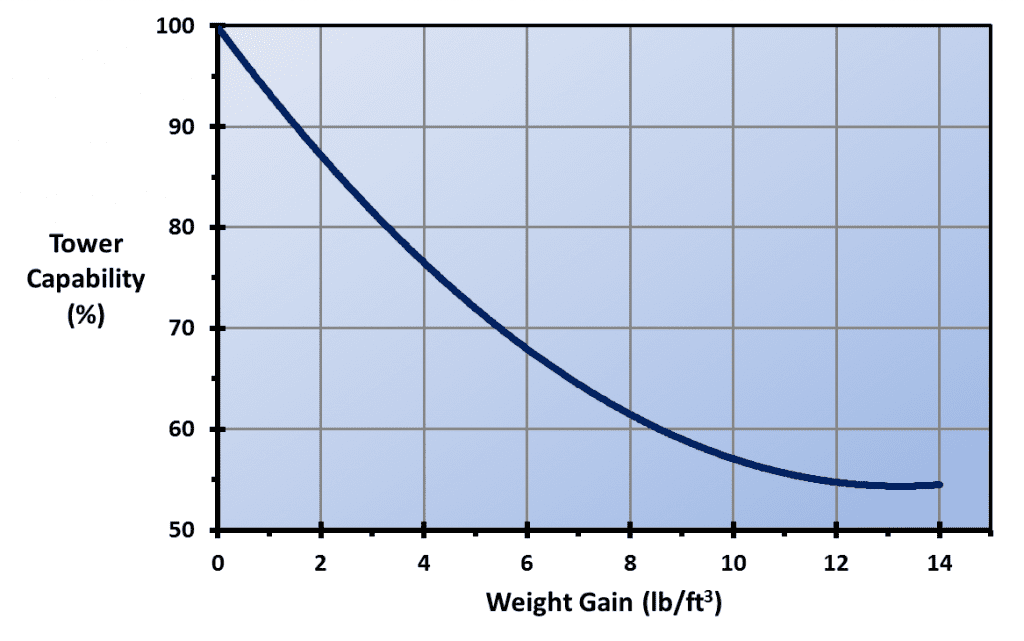
A critical aspect of cooling tower operation and cooling water treatment is that evaporation causes an increase in dissolved and suspended solids concentrations. The common vernacular in the industry for the concentration factor is cycles of concentration (COC). COC can be monitored by comparing the levels of a very soluble ion, such as chloride, in the recirculating (R) water and makeup (MU). However, this procedure requires laboratory analyses. A typical substitute is online-specific conductivity monitoring of the two streams, which can be programmed to automatically bleed off some recirculating water when it becomes too concentrated. A common COC range is 4 to 6. The water savings by increasing blowdown beyond this range become minimal, as the graph below clearly shows.
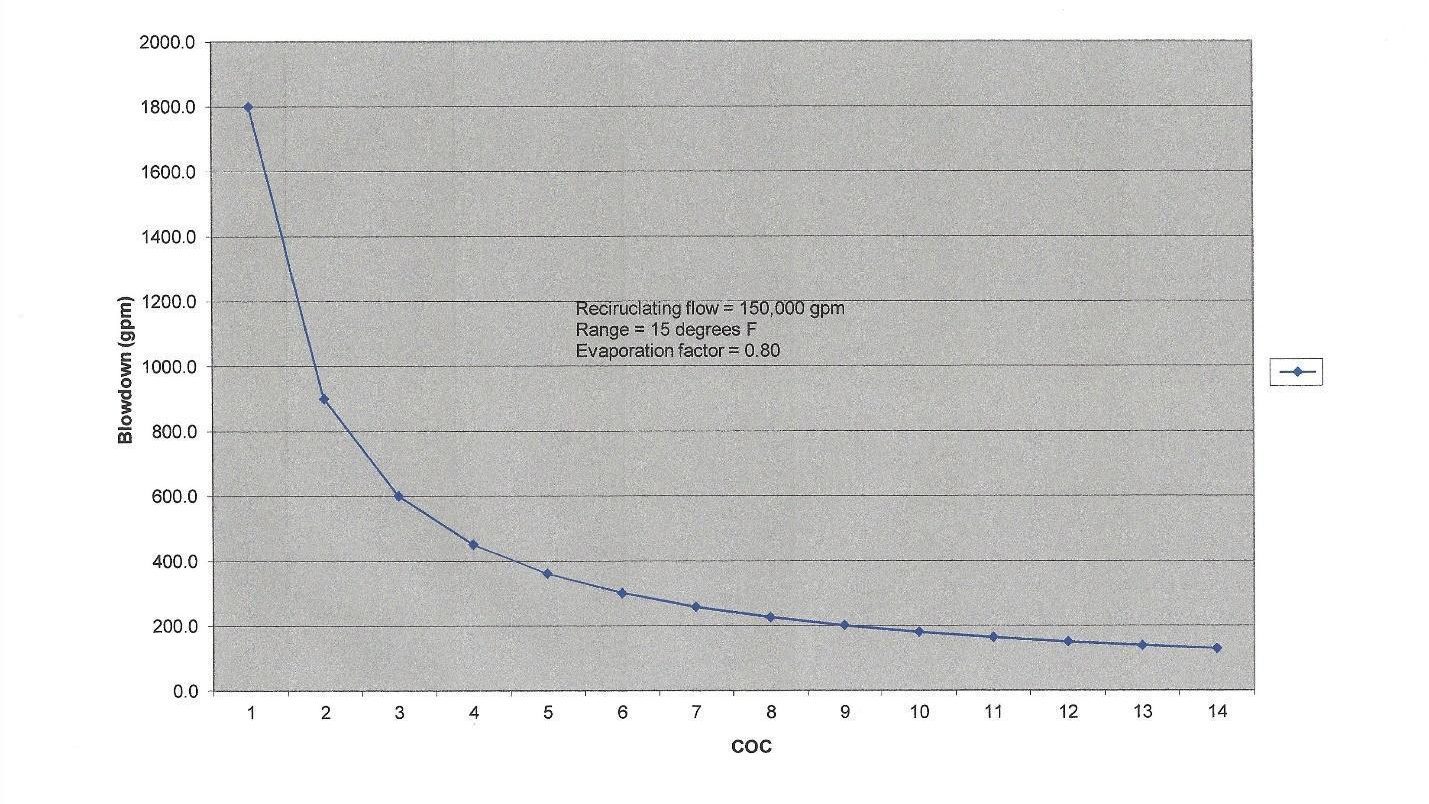
COC, or perhaps more accurately, allowable COC, varies from tower to tower depending upon many factors including makeup water chemistry, heat load, effectiveness of chemical treatment programs, and possible restrictions on water discharge quality or quantity. In arid locations, COC may need to be high, but chemistry control becomes more difficult.
A straightforward set of equations is available to reasonably calculate the blowdown (BD) and makeup requirements from a tower when the evaporation is known and allowable COC has been determined.
BD = E/(COC – 1) | Eq. 6-2
MU = E + BD + D + L | Eq. 6-3
Regarding Equation 6-3, it has already been noted that some water escapes the tower as drift (D), but in towers with state-of-the-art drift eliminators, drift is quite small. Leaks in the cooling system are referred to as losses (L), which also contribute to blowdown. In older systems, leaks from corrosion of piping and other equipment may be significant.
Chapter 7 discusses water treatment technologies to control scaling and fouling in cooling towers and the systems they supply, but the following section outlines a physical method for suspended solids control.
Sidestream Filtration
Suspended solids enter cooling towers via the makeup water, and as dust and other airborne debris. A prime example of the latter in many locations is cottonwood seeds, which readily foul in-line equipment filters. As with dissolved solids, particulates “cycle up” in the recirculating water, and can settle in low velocity areas of the cooling system, including the tower basin.
Sidestream filtration may effectively remove suspended solids in both open and closed cooling systems. Figure 6.25 shows two common filter locations.
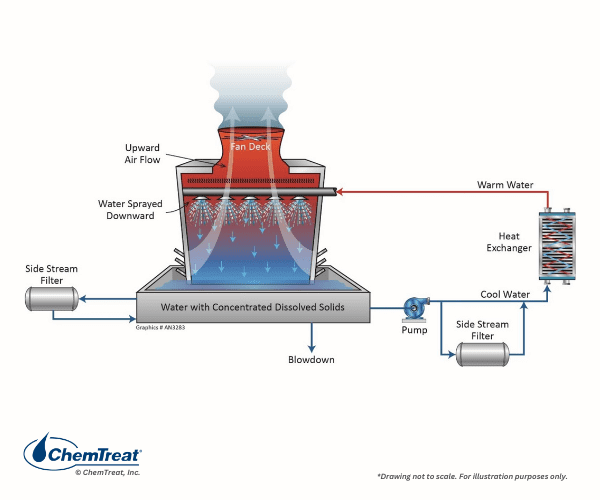
The table below outlines four of the most common sidestream filter technologies.
Table 6-2. Sidestream Technologies
Source: Sidestream Filtration for Cooling Towers (energy.gov)
| Filter Type | Particle Removal Level | Basic Filtering Mechanism | Applications | Notes |
| Centrifugal Separator | 40–75 microns, fine to coarse inorganics with a specific gravity of 1.62 or greater | High velocity water is fed in a circular pattern that [forces] heavier particles down and out of the system | Best for removal of large, heavy particles | Minimal maintenance required |
| Automatic Screen Filter | Down to 10 microns | Water flows through a rigid screen, where large particles are trapped and then removed from the system | Best for systems that cannot be interrupted such as industrial processes and hospitals | Self-cleaning mechanism is automatic and requires little down time |
| Plastic Disc Filter | Down to 10 microns | Grooved, stacked plates trap particles as water moves through the discs | Appropriate where removal of both solids and organics are required | Self-cleaning mechanism is automatic and requires little down time |
| Sand Filters | Down to 10 microns for pressure sand filters; to 0.45 microns for high-efficiency sand filters | Layers of granulated sand trap particles | Best for applications that require removal of fine and low-density particles | Supplemental chlorine feed may be needed because sand filters can promote microbiological growth |
A pressure sand filter is shown below.
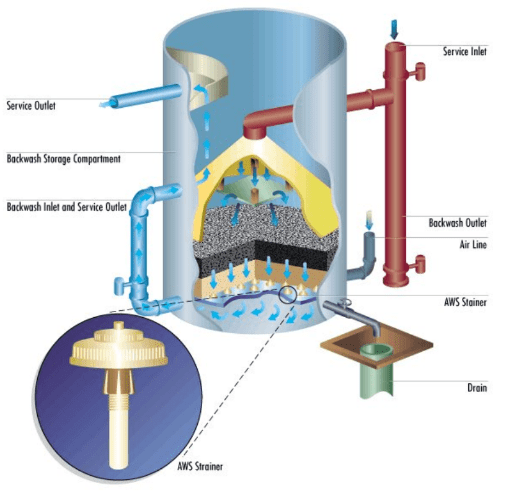
Choosing the optimum filtration method may require a careful evaluation of particulate sources and extent of ingress.
A Note About Tower Performance Monitoring
Besides periodic visual inspections of cooling tower components for scaling, fouling, or damage, regular operating data analyses are important to ensure reliable tower performance. The list below outlines important data for each test.
- Wind speed and direction (Measured upwind of the tower in an unobstructed location)
- Return water flow in the primary header(s) to the tower. (This measurement was once difficult, but new flow meter technology allows much greater accuracy.)
- Cold water temperature at the circulating pumps in the tower basin and warm return water temperature at the inlet to the tower.
- Power consumption at pumps and fans.
- Psychrometric calculations that incorporate air temperature and humidity to calculate wet-bulb temperatures and provide other data for test code requirements.
Also recommended are periodic comprehensive evaluations by a reputable vendor. The Cooling Technology Institute can supply information regarding qualified firms.
Cooling Tower Alternatives
Several cooling tower modifications or outright alternative technologies have emerged over the decades. While a detailed discussion of these alternatives is beyond the scope of this chapter, an overview of some of the most important appears in the following sections.
Evaporative Condensers
An evaporative condenser (see Figure 6.27 below) consists of a conventional cooling tower with a naked (no outer shell) heat exchanger located inside the tower.
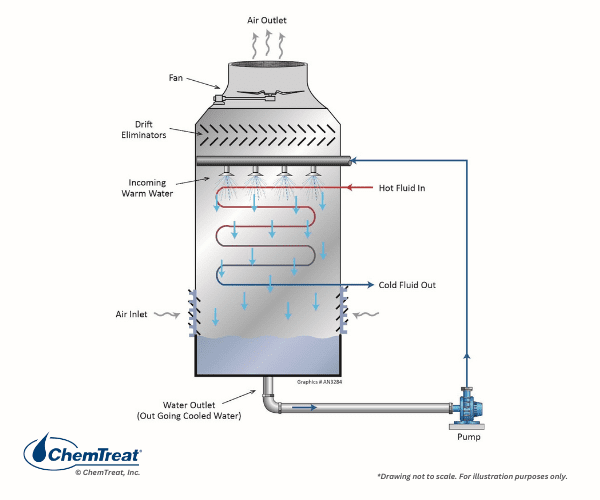
Placing the condenser inside the cooling tower reduces the space required to cool the refrigerant in a chiller air-conditioner system. However, this arrangement presents challenges in minimizing corrosion and deposition on the tubes. Heat exchanger cleaning can be quite difficult, especially interior tubes within a bundle.
Important with these units are proper spray header design and positioning to evenly distribute the water over the tubes. Regular inspection and maintenance of spray headers nozzles is important.
A variation of this design is the wet-surface air cooler (WSAC®), as shown in Figure 6.28.
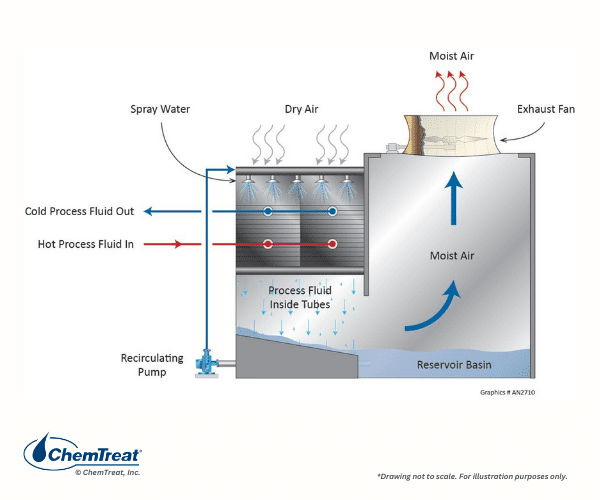
Cooling water and air travel co-currently in this design, where the configuration provides a large surface area for cooling and reduces the amount of water lost to evaporation.
Air Cooled Condensers
Air-cooled condensers (ACC) were originally designed for power plant turbine exhaust steam cooling in arid applications but are now being adopted in other locations.

These units utilize ambient air for cooling, and thus steam condensation is by sensible heat transfer only.
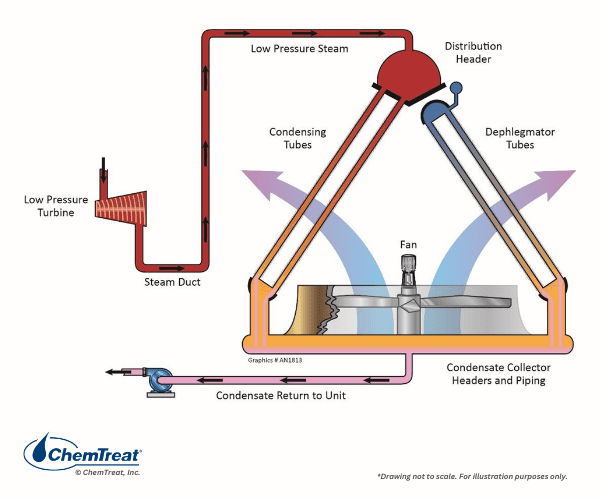
As Figure 6.30 illustrates, ACCs must be quite large to provide the necessary cooling. This is easily understood by comparing the density and heat capacity of air to water. Furthermore, ACCs can only cool steam to temperatures approaching the ambient-air dry bulb, not wet bulb. Thus, during warm months the turbine efficiency is significantly lower than for a comparable unit with a cooling tower and water-cooled condenser.
A smaller cousin to this process, fin-fan cooling is utilized at many plants for conditioning of liquid water and process streams.

These coolers typically have many small diameter tubes, which can become clogged with solids if chemistry control is inadequate.
Air Washers
Air washers are present in some industries, e.g., textile spinning mills, to control air temperature and humidity, and to maximize production and remove particles from the air.
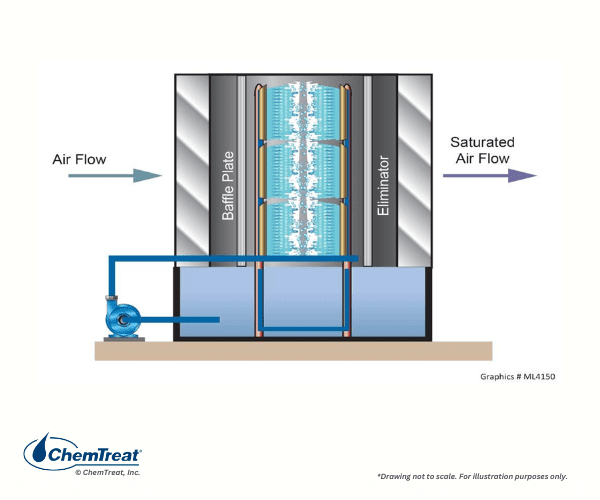
During summer when evaporation is enhanced, the units may operate as a once-through system. In winter, the process may revert somewhat to open recirculation like a cooling tower, which requires blowdown.
Closed Loop Water Cooling
Many plants have numerous heat exchangers that are embedded within one or more closed cooling water systems. These auxiliary “closed” heat exchangers reject heat to the primary open recirculation cooling system.
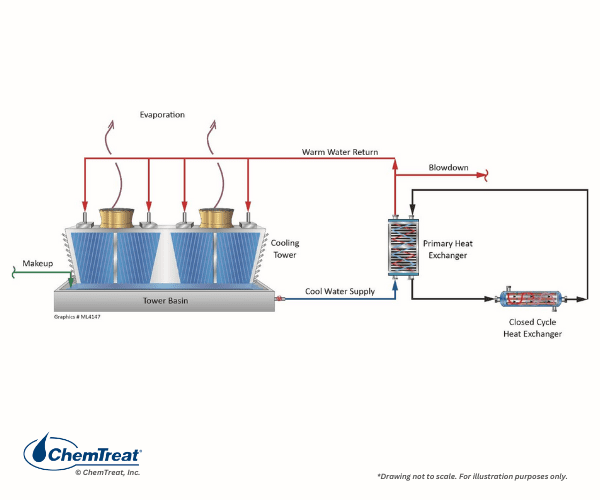
The term closed cooling water system is somewhat of a misnomer, as virtually all systems have leaks or small losses somewhere that require makeup. (If serious corrosion has occurred, these losses may be significant.) A closed system is basically defined as a loop which has little or no evaporation, and where makeup requirements do not exceed
5–15% of volume each year.
Systems are often designed with a head tank for water makeup and to absorb volume changes per temperature and load fluctuations. This arrangement can allow oxygen to enter the cooling water, which, of course, influences the corrosion potential. Some closed systems may have a pressurized expansion or bladder tank to maintain constant water pressure. Makeup vessels or tanks are often located at the highest point in a closed loop to serve as an air release outlet for non-condensable gases that might otherwise accumulate in the system and can cause corrosion and pump cavitation.
Examples of industrial and commercial closed cooling applications include:
- Pump bearings
- Lubricating oil
- Automated welders
- Metal casting coolers
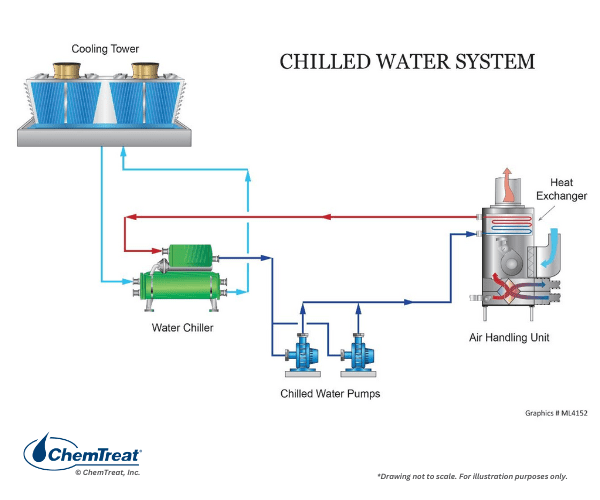
- Chilled water systems
The figure below shows the outline of a basic chilled water system.
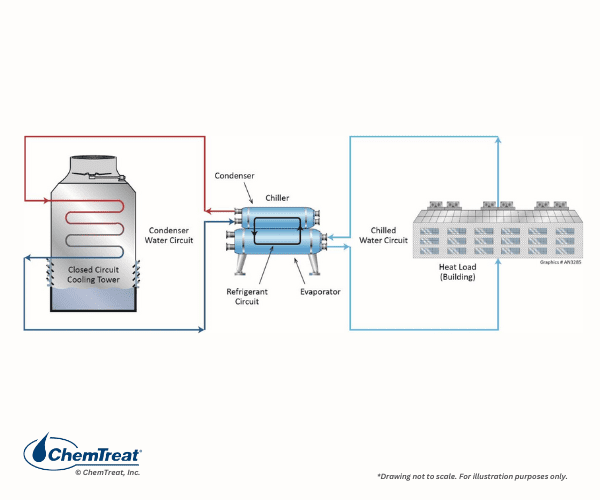
A variation of this design is shown below, with a closed cooling tower serving as the primary cooling circuit.
Most closed systems are equipped with a small side-stream pot feeder having inlet and outlet isolation valves for batch chemical feed. An enhancement is a combination of feeder and side-stream filter to remove those metal corrosion products that inevitably form during operation. A filter can be particularly valuable for some applications. An example is the automated elders at automobile assembly plants, which have small-bore, serpentine cooling lines in the welder heads. Particulate accumulation (and fouling or scaling) can be very problematic.
As will be covered in greater detail in Chapter 7, some closed water loops require high-purity makeup water, i.e., condensate. An example is a steel mill continuous casting cooler where the heat transfer rate is extremely large (106 Btu/ft2/hr). Corrosion or fouling that restricts heat transfer can be extremely detrimental and dangerous if “breakout” of molten steel occurs during the casting process.
Thermal Energy Storage Systems
A technique utilized at some municipal central heating and cooling facilities is thermal energy storage (TES).
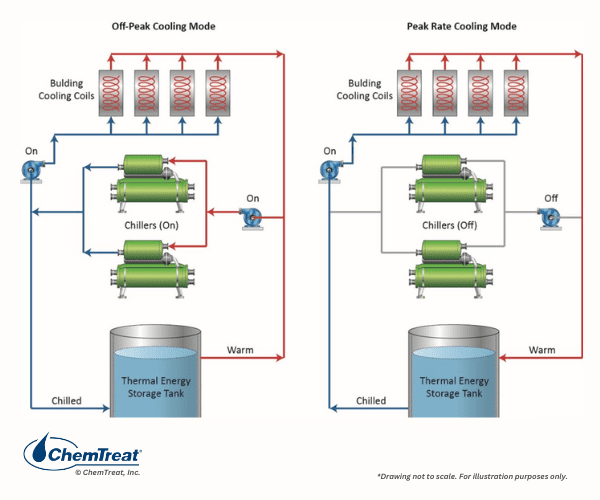
The chilled media may be water, ice, or even frozen brine, with storage capacities perhaps in the millions of gallons. The water is chilled at night during off-peak electrical demand when power costs are lower.
Hot Water Closed Systems
Hot water closed loops are widely employed for building heat. A number of configurations are possible, many with water return to the heater, which improves efficiency. Often at large industrial facilities hot water is utilized for energy transfer between industrial processes. For example, in ethylene cracking units a closed heat exchanger quenches the cracked ethylene gas, and then transfers the heat to incoming hydrocarbon feed stock. This particular thermal loop is termed a “Hot Water Belt.” The cooled water from the hydrocarbon heat exchanger returns to the ethylene cracker.
Heat Exchangers
Earlier sections of this chapter examined a very large heat exchanger at many plants, the cooling tower. And Chapter 4 covered another set of large heat exchangers, steam boilers. In the next sections, we will examine other water-cooled heat exchangers, and fundamental heat transfer properties.
Heat transfer is often described as the conveyance of energy and entropy from one point to another. When designing a heat transfer system, engineering and construction costs must be balanced against operating and maintenance costs. Sometimes expensive alloys or generous equipment size are worth the extra cost to prolong unit life and boost efficiency. In other cases, simple materials and design may be perfectly satisfactory.
Heat Exchanger Equipment
Perhaps the most common heat exchanger design is the shell-and-tube configuration. Shown below is a U-bend, two-pass exchanger.
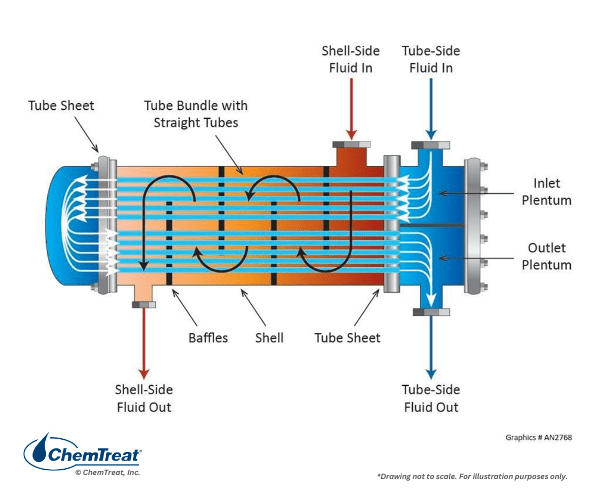
Such exchangers are common for liquid-to-liquid heat transfer when only one or neither of the fluids is water. This handbook, with its focus on water treatment, considers exchangers with water as the primary coolant, and where the water flow is through the tubes with the process fluid on the external tube surfaces. The design in Figure 6.37 is co-current with the coolant and process fluid flowing in the same direction. Note the baffle plates in the exchanger to enhance flow mixing and heat transfer. Two-pass heat exchangers are popular because they can provide greater cooling in a smaller amount of space than single-pass coolers.
Shown below is a counter-flow heat exchanger.
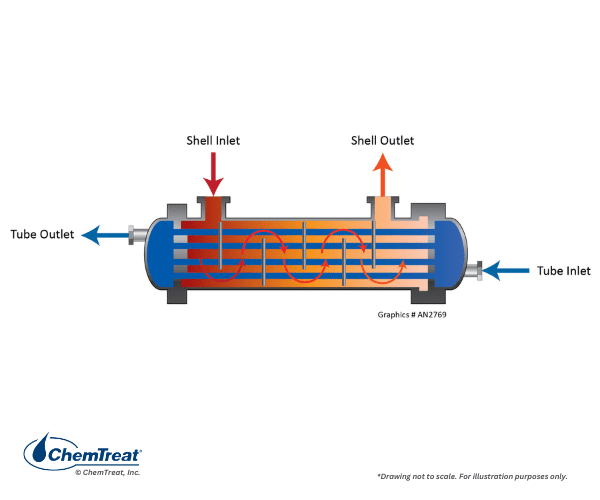
This design is often preferred because of lower thermal stress on the equipment, as the cooling water warms considerably before entering the highest-heat zone.
An interesting variation on this design is the steam surface condenser, which were so prominent at large coal and nuclear power plants, and are still prevalent at many combined cycle power plants.
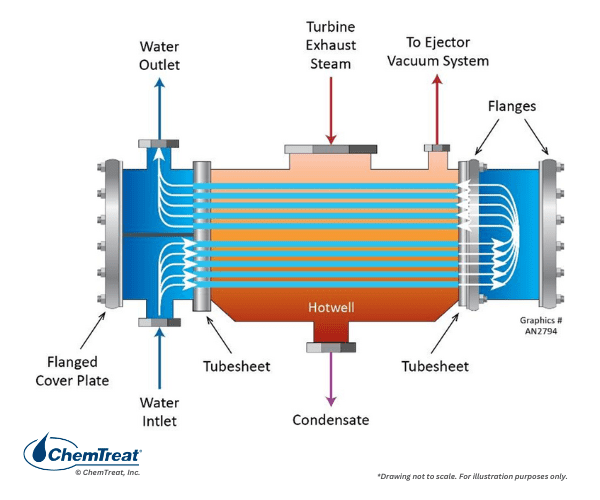
The incoming turbine exhaust steam usually is (and should be) of 90% or greater quality. The heat exchanger, with thousands of tubes, converts the steam to liquid for return to the boiler. Condensation improves the thermodynamic efficiency of the power generation process by nearly a third. However, condensation generates a very strong vacuum when the steam collapses to water. The strong vacuum pulls in air at even the tiniest openings in the condenser shell or other spots. If the air is allowed to accumulate, it will coat the tubes and greatly reduce heat transfer. Thus, surface condensers are typically equipped with an air removal compartment that is continuously exhausted by vacuum pumps.
Older coal-fired plants often had vertically aligned shell-and-tube exchangers for feedwater heating. This orientation is practical where horizontal space is limited.
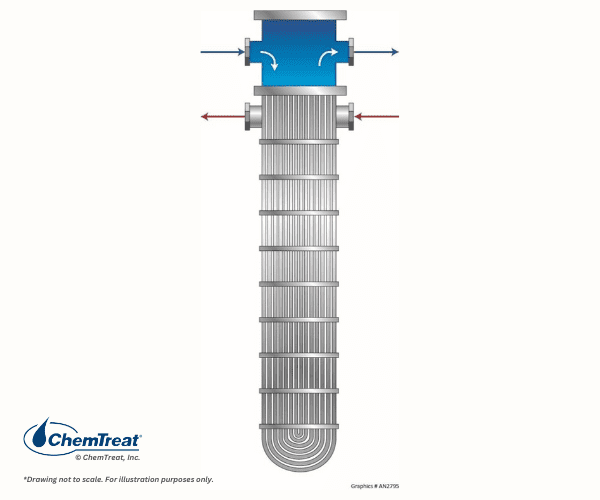
Suspended solids in the water may accumulate at the bottom of a vertical heat exchanger if flow is insufficient to keep solids in suspension. Periodic removal of the deposits may be necessary to prevent tubes from becoming blocked with material.

IDENTIFYING AND ADDRESSING CONDENSER PERFORMANCE ISSUES AT A COAL-FIRED POWER PLANT
Heat Exchanger Materials
For water cooled shell-and-tube heat exchangers, the shell is often carbon steel, with higher-grade materials for the tubes. In a steam condenser, potential tube materials include admiralty brass (70% copper, 29% zinc with minor additional elements), 90-10 copper-nickel, 70-30 copper-nickel, and more recently the 300-series stainless steels. For systems with cooling water that has high chloride concentrations, titanium, or super-ferritic stainless steels such as SeaCure® are common tube materials. Heat exchangers in refineries and chemical plants may require specialty alloys due to specific corrosive agents and high temperatures within the process streams. Sulfides can be quite corrosive to many metals. Strong acids or caustic may require special handling. The list goes on and on.
Other Heat Exchanger Designs
Another common design is the plate-and-frame heat exchanger.

These units offer a smaller footprint and lower cost than shell-and-tube exchangers. The figure below illustrates a basic flow path.
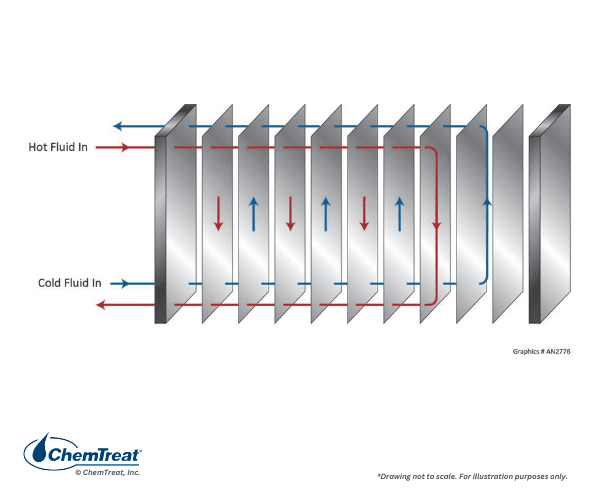
A drawback is that the tightly-spaced plates provide locations for low fluid velocities that allow suspended solids to settle. Some exchangers may have corrugated plates to enhance fluid mixing, which can present cleaning challenges that require disassembly of the exchanger.

Stainless steel is a common material for plate exchangers, but more exotic materials may be required in high-stress or corrosive applications.
Other heat exchangers may be spiral or helical. A diagram of each is shown below.
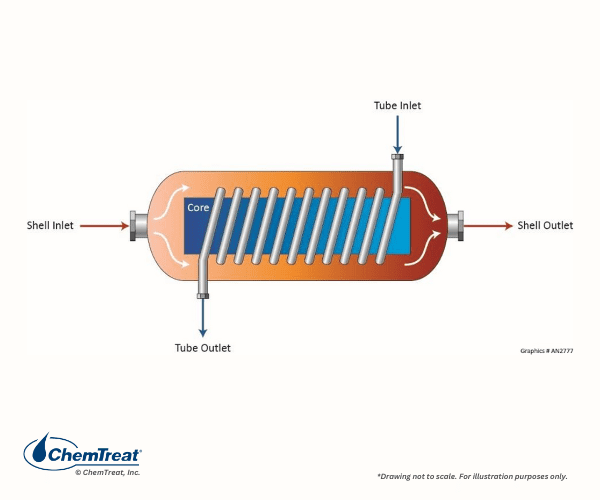
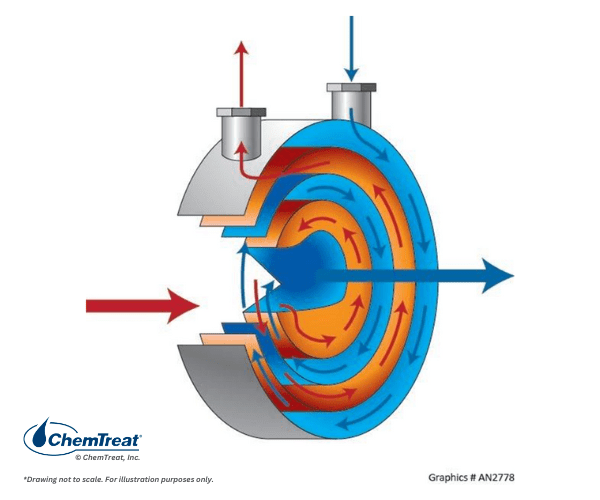
These heat exchangers are employed for specialty applications not covered in this handbook.
The above figures illustrate heat exchangers that provide a physical boundary between two fluids. In some applications, no boundary exists. A primary example is direct steam injection. The steam is then recovered as condensate further along in the process. However, the condensate may contain any number of impurities that require removal before return to the boiler.
Heat Transfer Fundamentals
The three general modes of heat transfer are convection, conduction, and radiation. These are discussed in Chapter 4. For most of the heat exchangers outlined above, conduction and convection are the primary heat transfer methods. The mathematics of heat transfer can be quite complicated, especially when designing systems. However, a great deal of understanding is possible from straightforward calculations, “When heat flows from one fluid to another through a solid retaining wall, the total amount of heat transferred may be expressed as follows:
(Q/t)total = U*A*ΔTtotal | Eq. 6-4
- Q/t is heat transfer per time, with the common English units of Btu/hr.
- U is the heat transfer coefficient (Btu/(hr) (ft2) (o F)
- A is the surface area (ft2) of the tubes or plates across which the heat is transferred.
- ΔT is the temperature difference between the two fluids. This is usually calculated as the “log mean temperature difference” (LMTD), which more accurately accounts for the change in temperature of the two fluids along the length of the heat exchanger.
- LMTD = (Δt2-Δt1)/ln(Δt2/Δt1)
The key variable in Equation 6-4 is U. When fluid flows through a tube, pipe or along a plate, even if the bulk flow is turbulent, a thin, laminar sublayer forms at the material surface. This film influences heat transfer. Accordingly, for a clean surface, the equation for U becomes:
1/U = 1/h′ + 1/h″ + xw/k | Eq. 6-5
- h′ is the film coefficient for the first fluid (Btu/(hr) (ft2) (o F)
- h″ is the film coefficient for the second fluid (Btu/(hr) (ft2) (o F)
- xw is the tube wall or plate thickness
- k is the unit heat transfer coefficient of the material (Btu/(hr) (ft2) (o F/ft)
An excellent example of heat transfer from steam condensing on a 2”, Schedule 40 carbon steel pipe is outlined in Reference 11. All details are not repeated here, but worth noting is that h′ (water film) is given as 500 Btu/(hr) (ft2) (o F), h″ (steam side) is given as 2,000 Btu/(hr) (ft2) (o F), and xw/k is 0.00046 (hr) (ft2) (o F)/Btu, where k for carbon steel is listed as 26 Btu/(hr) (ft2) (o F/ft). Taking the inverse of the first two and adding these numbers to the third value (and adjusting slightly for internal and external pipe surface area differences) gives a U value of 346 Btu/(hr) (ft2) (o F). For this particular example, with a single pipe 10 feet long and LMTD of 91o F, per Equation 6-4 the heat transfer to the cooling water is 170,000 Btu/hr.
A key observation from this example is that the heat transfer through the steam-side and the pipe wall are roughly equivalent, but that heat transfer through the water film is considerably lower. Thus, heat exchangers are often designed to maximize turbulent flow (within constraints on pumping requirements and metal resistance to erosion-corrosion) to reduce the laminar sub-layer thickness. For exchangers with liquid on both sides of the tubes or plates, the film heat transfer resistance increases. These factors are very important when designing the unit. If the process fluid is something other than water or steam, the calculations become more complex.
A very important criterion for selecting heat exchanger materials is the thermal conductivity. The data below offers some selected values from common heat exchanger materials.
Table 6-3. Thermal conductivities of some common heat exchanger materials.
Information provided by Dan Janikowski, Plymouth Tube Company per Heat Exchange Institute (HEI) data.
| Metal | Thermal Conductivity at 68o F (or near)(Btu/(hr) (ft2) (o F/ft) |
| Carbon Steel | 27.5 |
| Admiralty Brass (70 Cu, 29 Zn) | 64 |
| 90-10 Cu-Ni | 26 |
| 70-30 Cu-NI | 17 |
| 304 and 304L Stainless Steel | 8.6 |
| 316 and 316L Stainless Steel | 8.2 |
| Titanium (Grade 2) | 12.7 |
The table illustrates the large variety in thermal conductivities, and it clearly illustrates the much higher conductivity of admiralty brass as compared to carbon steel and especially stainless steel. (A caveat exists in this regard, as will shortly be outlined.) For this reason, in the middle of the last century, admiralty brass was a common selection for condenser and feedwater heater tubes in coal-fired power units. Thick tube walls were possible; designed to provide long service life. However, it became increasingly apparent that gradual copper corrosion allowed transport of the corrosion products to steam turbines, which deposited on the turbine blades and reduced efficiency. Many plant staffs replaced the admiralty tubes with stainless steel to eliminate this problem. Unfortunately, when this modification was made in some condensers, the stainless steel tubes began to suffer from water-side microbiologically-induced corrosion (MIC). Copper ions that leached from the admiralty tubes were toxic to microbes, whereas the steel did not offer the same protection. This is another example showing the importance of matching metal corrosion characteristics with process conditions and will be covered in further detail in Chapter 7.
The caveat mentioned above is that an oxide layer develops on many metals during service. The layer may be more or less protective depending on the environment and the metal. Copper alloys form an oxide patina, which is considerably more insulating than the base metal. This is another factor that must be considered during heat exchanger design.
Expanding on Equation 6-5, the equation below illustrates the influence of deposition on either side of the metal surface.
1/U = 1/h′ + 1/h″ + xw/k + 1/h′d + 1/h″d | Eq. 6-6
The last two terms account for deposition or film formation on each side of the tube wall or plate.
Mineral and microbiological deposits have low heat transfer coefficients as shown in the table below.
Table 6-4. Thermal conductivity of three common water-side deposits.
Information provided by Dan Janikowski, Plymouth Tube Company.
| Foulant or Scale | Thermal Conductivity (Btu/(hr) (ft2) (o F/ft) |
| Calcium carbonate | 1.25 |
| Silica | 0.8 |
| Biofilm or stagnant water | 0.36 |
Even a thin deposit layer will significantly reduce heat transfer. Note the confirmation of the insulating effect of a stagnant water layer. Porous biofilms can induce under-deposit corrosion, which may lead to premature failures and unit outages.

IMPROVING HEAT EXCHANGER PERFORMANCE AND RELIABILITY AT A CHEMICAL PLANT USING CHEMTREAT FLEXPRO®
Additional Heat Balance Calculations
When heat is exchanged between two liquids with no phase change by either, the amount of heat lost by the warmer liquid is balanced by that gained by the coolant. The energy exchange is all sensible heat transfer, and, excluding minor losses, can be described by the following standard equation.
Q/t = ṁ *Cp*∆T | Eq. 6-7
- ṁ = process flow (lb/hr)
- Cp = specific heat of the fluid, for water Cp=1.0 (1 Btu/lb o F)
- ∆T = o F
If one of the fluids condenses from a vapor, then the latent heat (H) is also transferred, and Equation 6-7 expands to:
Q/t = ṁ*Cp*∆T + ṁ*ΔH | Eq. 6-8
The importance of latent heat recovery continues to have a growing influence on plant design. Consider the many coal-fired units constructed in the last century. The fundamental process involves the production of superheated steam to drive a turbine generator. (See Chapter 4.) The turbine exhaust steam is cooled to liquid in the condenser for return to the boiler. Condensation significantly improves the net thermodynamic efficiency of the unit, but most of the steam’s latent heat is still lost to the cooling water. The best drum units are only capable of approximately 35% net efficiency, and even the most modern ultra-supercritical units can achieve at most 45% efficiency. Co-generation and combined heat and power (CHP) units may achieve up to 80% net efficiency. A common co-gen configuration uses superheated steam to drive a turbine for electricity production, but with steam extraction before complete loss of superheat. The extracted steam feeds process heat exchangers, in which the latent heat is also utilized. This scheme is becoming increasingly common at industrial facilities to produce electricity internally and maximize steam heat efficiency.
Heat Exchanger (HX) Performance Monitoring
Specification sheets are typical for new heat exchangers, and an example from the Tubular Exchange Manufacturers Association (TEMA) is shown below.
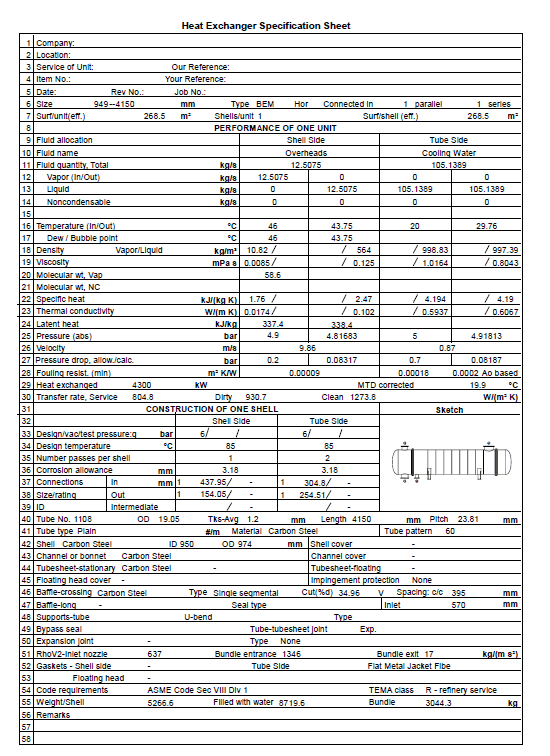
When units are placed into service, it is important to collect all relevant operating data, as this is when the exchanger is at its most pristine with maximum heat transfer efficiency. Typically, the data will not exactly match the specification sheet, but it serves as the baseline for the future. Important data for inlet and outlet streams is outlined in Figure 6.46 and includes the following:
- Temperatures and pressures
- Flow rates
- Densities and viscosities
- Specific heats
Also highly important are accurate process flow diagrams (PFDs) and process and instrument diagrams (P&IDs) that clearly outline the equipment and piping within a unit operation, including flow rates, pressures, and temperatures at full capacity. For plants such as refineries, chemical manufacturing, and other similar facilities, a huge number of diagrams is necessary. A frequent mistake at many plants has been lack of documentation for piping and equipment modifications. This becomes even more problematic when changes are made to underground piping without proper documentation.
Common issues that affect heat exchangers materially and/or performance-wise include:
- Reduced cooling water flow due to pump impellor degradation or other problems. Flow losses are often gradual and may be overlooked. Reduced flow can increase process temperatures that in turn cause corrosion or scaling problems on the process side, and influence deposition and corrosion on the cooling side.
- Ill-considered manual adjustments to HX inlet and outlet valve positions. A common complaint heard at plants when equipment performance suddenly becomes erratic is that a “gremlin” must have tampered with it. The gremlin, of course, is someone from the plant who changed a valve setting, modified a pump feed rate, or made some other change without informing anyone.
- Incorrect materials selection for the application
- Stainless steel is often selected in knee-jerk fashion during project design, only for rapid failures to be induced by a particular corrosive agent in the cooling water or process fluid. Chlorides can be quite corrosive to the austenitic stainless steels, especially if they concentrate under deposits such as those from microbiological fouling.
- Sometimes the material selected for the application may be suitable, but the fabrication methods are faulty. A classic example was installation of new 90-10 copper-nickel tubes in a steam surface condenser for a once-through application with raw water from a recreational lake. The tubes should have lasted for decades but failed within 18 months from through-wall penetrations because the fabricator utilized a lubricating fluid that contained sulfides.
- Poor or ineffective water treatment program selection or operation (covered in Chapter 7)
- Inadequate initial cleaning and passivation (covered in Chapter 7)
Another common error, and especially at plants with many heat exchangers, is to monitor all exchangers rather superficially and overlook data from individual units that suggest a serious problem. Allocating resources for personnel to better focus on heat exchanger operation can be beneficial in finding and correcting problems before they become major issues.

IMPROVED RELIABILITY AND PROFITABILITY AT A GULF COAST CHEMICAL PLANT WITH FLEXPRO® TECHNOLOGY
Tremendous advancements have been made in the development of online instrumentation and data-analysis algorithms to track equipment performance, provide readings to control room and auxiliary operators, and in some cases make automatic adjustments to equipment such as water treatment chemical feed pumps. The costs can often be justified many times over by early identification of unfavorable conditions to allow prompt corrective action that prevents efficiency loss or even equipment failure.
Important information that data systems collect, include:
- Cooling water and process fluid inlet and outlet temperatures.
- A very helpful calculation is the approach temperature, which is the process temperatureout – cooling water temperaturein. In many heat exchangers, the approach temperature should normally remain relatively constant. A common rule-of-thumb range is 8–20°F. A large increase suggests flow or fouling issues. The approach temperature of a particular heat exchanger is sensitive and varies with the heat load. Therefore, trending of approach temperature measurements is best made under the same or nearly the same heat load conditions, otherwise the data must be adjusted, i.e., normalized, based on heat load.
- Thermocouple and thermistor readings that activate preset alarms, permit remote monitoring, and allow quick response by personnel.
- Flow rates and pressures (and pressure differentials). These measurements will identify both short-term and long-term equipment problems such as pump malfunction, and tube blockage from large solids or foulants.
- Fluid levels and level controls. These measurements are important in a variety of plant processes from raw material storage to heat exchangers in which a phase change occurs.
- By calculation, heat load and heat transfer coefficient data. Data that trends downward suggest efficiency degradation. Another calculation, terminal temperature difference (TTD), is valuable for steam surface condenser performance monitoring and is discussed in greater detail below.
- Water chemistry, including pH, specific conductivity, and other parameters depending on the individual application. These issues are covered in Chapter 7.
The next and final section provides additional details regarding steam condenser performance monitoring.
The power industry continues to undergo much change. Coal-fired power generation has been on a steep decline in many areas of the world due to climate concerns and efforts to reduce CO2 emissions. Besides renewable energy technologies, combined cycle power generation is taking the place of coal units. A significant portion of combined cycle power is produced by heat recovery steam generators (HRSGs) and accompanying steam turbines, whose heat source is combustion turbine exhaust gas. While ACCs are becoming more common for steam turbine exhaust cooling, many HRSGs still have water-cooled condensers. For plants with these condensers, several measurements are very effective for monitoring performance.

PROTECTING THE WATER/STEAM CHEMISTRY OF CO-GENERATION AND COMBINED HEAT AND POWER PLANTS
TTD and Cleanliness Factor
The three critical temperature measurements for evaluating condenser performance are cooling water inlet and outlet, and condensed steam that collects in the hotwell. Of course, the inlet and outlet temperatures will always undergo seasonal changes, which can mask problems. The key measurement is the TTD, which for condensers is the condensed steam temperature minus the cooling water outlet temperature. When TTD data are collected over time at consistent unit load, preferably full load, the readings should remain steady if the condenser is operating properly. Factors that impede heat transfer and induce a TTD rise are water-side fouling or scale formation, or excess air in-leakage on the steam side.
Years ago, the HEI developed calculations that utilize the above-mentioned temperature readings along with condenser tube material data, total number of tubes and number of plugged tubes, tube dimensions including length, diameter, and wall thickness, number of passes (normally 1 or 2), and cooling water flow rate to generate what is known as the condenser cleanliness factor (CF). The program calculates a design heat transfer coefficient (Ud) and an actual heat transfer coefficient (Ua), where Ua/Ud * 100 is the CF in percentage. Theoretically, the CF should be around 85% for a clean condenser with no plugged tubes, but the value is particular to each individual unit. Therefore, data should be collected at condenser startup or after tube cleaning to serve as the baseline for continued monitoring. The program can be very effective at detecting the gradual loss of heat transfer from water-side scaling or microbiological fouling, to excess air in-leakage on the steam side.
Vacuum and Backpressure
The enormous decrease in volume when turbine exhaust steam converts to water in the condenser induces a very strong vacuum. In wintertime with cold cooling water, the vacuum may reach around 1 inch (Hg) above absolute (-29.92 inches gauge pressure at sea level). This value is known as the condenser backpressure. It will rise during the spring and summer, which is one reason why power plant cooling water systems often have multiple pumps, such that the cooling water flow rate can be increased during the warm weather months. Backpressure is a valuable tool for tracking condenser performance, recognizing that it will rise by perhaps two to four inches from winter to summer. If condenser fouling induces higher backpressures, fuel requirements and costs may increase dramatically. Beyond that issue, turbines are designed to trip offline if the backpressure rises much higher than normal summer maximum. A unit trip during a summer peak can be enormously expensive.
As an additional note, it is the strong vacuum generated in the condenser that pulls in ambient air at structural irregularities or failures at the condenser or any surrounding auxiliary systems. A common occurrence is a sudden crack in the condenser shell. If the air ingress is greater than can be handled by the air-removal system, the excess air will form pockets in the condenser and significantly restrict heat transfer. A good CF program will often detect such problems very quickly.
Circulating Pump Discharge Pressure
Condenser fouling or scaling impedes fluid flow and will appear as an increase in circulating pump discharge pressure. Generally, the discharge pressure ranges from 20 to 80 psi depending on the equipment served and elevations. Conversely, over time circulating pump flow tends to decrease due to impellor degradation. For a long time, accurately measuring such large flows in the field was nearly impossible, but new instrumentation offers solutions to that issue.
Conclusion
This chapter outlined important fundamental heat exchanger aspects at industrial facilities and power plants. Many exchanger varieties are available, with numerous metallurgies possible. These details are very important when selecting and implementing chemical treatment programs to protect cooling systems from corrosion, scaling, and microbiological fouling. For this reason, industrial plants and other facilities can greatly benefit from cooling tower treatment. We take up these chemistry issues in the next chapter.
Speak With a ChemTreat Expert Today
ChemTreat is committed to providing expert water treatment solutions. We maintain environmental and quality management systems certifications to prove our expertise as a water treatment company and provide customized solutions for every client. If you are looking to increase the efficiency and reliability of your water cooling systems, contact ChemTreat today to get in touch with a local representative to learn how you can transform your operations.
References
- J. Hensley, ed., Cooling Tower Fundamentals, 2nd Edition, SPX Cooling Technologies, Inc., Overland Park, Kansas, 2009.
- Buecker, B., and R. Aull, “Cooling Tower Heat Transfer Basics – Part 2”; Hydrocarbon Processing, August 2020.
- Post, R., Emery, K., Dombroski, G., and M. Fagan, “Effectively Cleaning Cellular Plastic Cooling Tower Fill”; from the conference proceedings of the 33rd Annual Electric Utility Chemistry Workshop, June 11-13, 2013, Champaign, Illinois.
- Characklis, W. G., Biofilms, John Wiley & Sons, 1990.
- Aull, R., and R. Post, “Chemical Treatment Methods and Fill Selection to Minimize Scaling/Fouling in Cooling Towers”; EPRI Cooling Technology Seminar, Pensacola, Florida, 2012.
- Monjoie, M., Russell, N., and G. Mirsky, “Research of Fouling Film Fill”; Cooling Technology Institute, TP93-06, New Orleans, Louisiana, 1993.
- Makowski, et al., “Apparatus and Method of Removing Microfouling from the Waterside of a Heat Exchanger”. US Patent 5,558,157. 1996.
- Potter, M.C., and C.W. Somerton, Schaum’s Outline of Theory and Problems of Thermodynamics for Engineers; McGraw-Hill, 1993.
- B. Buecker, “Solutions to Challenging Power Plant Flow Measurements”; Power Engineering, December 2018.
- Kreith, F., and R.M. Manglik, Principles of Heat Transfer, 8th Edition. Cengage Learning.
- M. Peters, Elementary Chemical Engineering; McGraw-Hill, 1954.
- B. Buecker, “Computer Program Predicts Condenser Cleanliness”; Power Engineering, June 1992.
- B. Buecker, “Dealing with Challenging Flow Measurement”; Industrial WaterWorld, (now Water Technology), May/June 2018.
About the Authors

Tom Nix
Senior Technical Staff Consultant
Tom Nix is a trusted technical expert with decades of industrial water treatment experience. Nix is well-versed in a wide range of applications, including influent clarification, softening, demineralization, reverse osmosis, cooling water treatment, low- and high-pressure boiler treatment, and wastewater treatment. He holds a B.S. in Environmental Biology from the University of Texas at Austin.

Brad Buecker
President of Buecker & Associates, LLC
Brad Buecker is president of Buecker & Associates, LLC, and most recently he served as Senior Technical Publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, and air quality control. Buecker has a B.S. in Chemistry from Iowa State University. He has authored or co-authored over 250 articles for various technical trade magazines and has written three books on power plant chemistry and air pollution control. He serves on the Editorial Advisory Board of Water Technology and is a member of the ACS, AIChE, AIST, ASME, NACE (now AMPP), and the Electric Utility Chemistry Workshop planning committee.
Acknowledgments
The ChemTreat Water Essentials Handbook would not have been possible without the contributions of many people. See the full list of contributors.
