-
USA - English
- Locations
- SDS Access
- CTVista®+ Login
Minimizing condensate and feedwater piping corrosion can be a critical issue for steam-generating systems. Corrosion of the (typically) carbon steel materials in these systems can induce direct equipment failures and can also release iron oxide particulates that transport to steam generators and cause boiler tube deposition issues.
The primary culprit of most feedwater corrosion is dissolved oxygen (D.O.). Oxygen is very aggressive towards carbon steel and often causes pitting corrosion. For that reason, low-pressure steam units are normally equipped with mechanical deaerators to remove most of the D.O. in the feedwater.
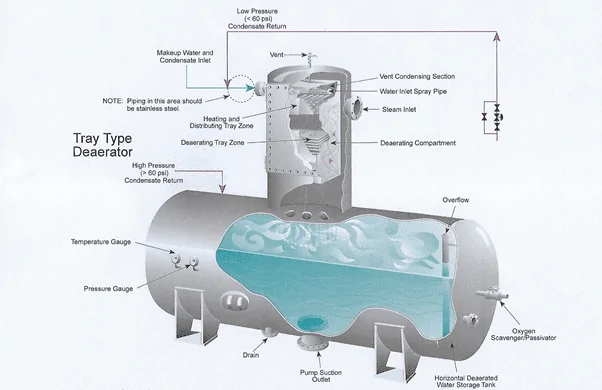
Figure 1. A tray-type deaerator. Steam counter-currently contacts inlet condensate. Heating and agitation within the deaerating compartment strip most of the D.O., which is vented.
A properly operating deaerator will reduce D.O. concentrations to 7 parts per billion (ppb). A common method of reducing D.O. concentrations to near zero is supplemental injection of a chemical oxygen scavenger/reducing agent in the dearator storage vessel or outlet line. Also included in the feedwater treatment program is ammonia or a neutralizing amine to minimize general corrosion.
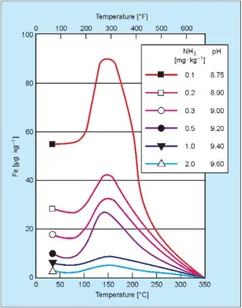
Figure 2. Influence of pH and temperature on iron dissolution from carbon steel. Source: Sturla, P., Proc., Fifth National Feedwater Conference, 1973, Prague, Czechoslovakia.
In the power industry, high-temperature, high-pressure steam generators require much higher purity makeup than low-pressure industrial units. However, for over three decades now it has been known that complete deaeration of high-purity feedwater leads to flow-accelerated corrosion (FAC). In this mechanism, iron gradually leaches out of the carbon steel at flow disturbances such as feedwater and economizer elbows, inducing gradual wall thinning until a rupture occurs. FAC can cause serious problems if not treated. Accordingly, unless the feedwater network contains any copper-alloy components (e.g., feedwater heater tubes) treatment programs for high-pressure steam generators call for a small D.O. residual in the feedwater. For virtually any unit, whether low- or high-pressure, feedwater iron monitoring provides critical data on the performance of the treatment program, where long-established guidelines suggest proper results when total iron concentrations are less than 2 ppb.
Grab sampling analyses can be very effective for tracking carbon steel corrosion. However, because most iron corrosion products exist as particulates, the standard analytical technique for dissolved iron analyses will be quite inaccurate. A digestion process is required to convert particulate iron oxides to dissolved iron, which can then be quickly analyzed via UV-VIS (ultraviolet-visible) spectrophotometry.
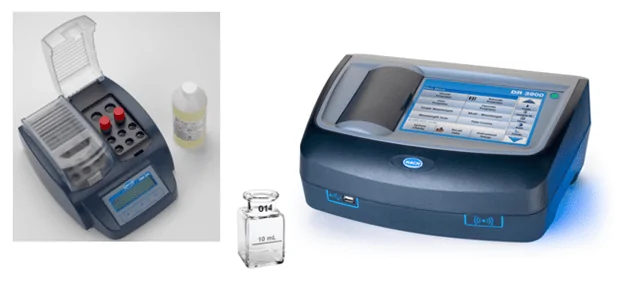
Figure 3. Combination reagent, digestion vials, and heater block (left); 1-inch sample cell (center) and spectrophotometer (right). Photos courtesy of Hach.
This technique can detect iron down to a concentration of approximately 1 ppb. For those desiring a more sophisticated approach, some companies have developed nephelometric methods that use light-scattering to track iron oxide concentrations on a continuous basis. These analyses can be very accurate but are site-specific and require individual calibration based on system requirements.
Please contact ChemTreat for assistance in designing a treatment program customized for your application. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides.
