Many industrial plants have water-cooled heat exchangers that serve a variety of purposes. The cooling water may be once-through or supplied by a cooling tower.
Why Is Scale Treatment Important?
Over time, exchangers can accumulate scale that reduces heat transfer. Heat transfer degradation can cost a plant money and may negatively influence process chemistry in some situations. So, it is prudent to have proactive procedures in place to monitor important heat exchanger data and ensure chemical treatment programs are performing effectively.
Even when using proper chemistry, periodic scale removal is often a necessity. Selecting the proper cleaning solution is very important, but frequently not given sufficient consideration.
The most common mineral deposit on the water side of heat exchangers is calcium carbonate (CaCO3). Most raw waters contain significant dissolved concentrations of calcium (Ca2+) and bicarbonate (HCO3–) ions, which, when heated, precipitate as shown in the following equation:
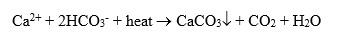
This is also the compound known as lime scale that forms in the hot water piping, faucets, and showerheads of many home plumbing systems.
Removing Scale from Heat Exchangers
Removing calcium carbonate from heat exchangers is relatively straightforward. Because CaCO3 is the salt of weak acid, any solution with even moderate acidity will dissolve the compound.
But this is where those unfamiliar with chemistry often go astray. Our team has directly observed situations where plant operators and technical personnel selected off-the-shelf muriatic acid when preparing cleaning solutions. Muriatic acid is the trade name for hydrochloric acid (HCl), and while it will easily remove calcium carbonate, the chloride ion can cause serious pitting and stress corrosion cracking of stainless steel.
Overfeeding HCl lowers pH, potentially damaging carbon steel piping and other equipment. Repeated use of this solvent can lead to heat exchanger failures.
Higher-grade options such as sulfamic and citric acids may be a much better (and not exorbitantly expensive!) choice. These compounds will remove not only calcium carbonate but also accumulated iron oxides, especially if the solution can be heated to approximately 100°F.
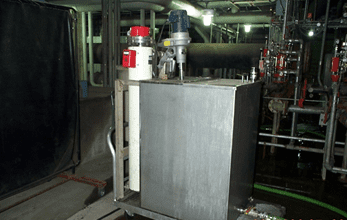
Figure 1. A wheeled cart with tank, tank mixer, and heater for preparation of heat exchanger chemical cleaning solutions. Photo courtesy of Brad Buecker.
Circulating the acid for a few hours (usually for no more than an eight-hour shift) is often satisfactory to remove CaCO3 and iron.
A note of caution: when calcium carbonate reacts with acid, carbon dioxide is released, potentially causing the solution to foam. Monitoring pump operation, particularly at the beginning of the process, is recommended to ensure the pump maintains prime.
In some applications, other deposition may occur. One possibility is calcium sulfate (CaSO4). This compound is the salt of a strong acid, and thus will not dissolve in acids like calcium carbonate will. One method to address issues such as these is chemistry that goes after the cationic portion of the deposit, in this case, the calcium ions. Careful preparation and implementation of cleaning procedures is necessary for minimizing potential corrosion of the base metal.
The Importance of Proper Disposal
In any cleaning process, disposing of the spent solution from the heat exchanger and piping should not be neglected. Simply dumping the material down a plant drain may violate your discharge permit. If possible, we recommend transporting the spent solution to your wastewater treatment facility for proper conditioning prior to discharge.
Please contact ChemTreat for help cleaning your heat exchangers. We have the necessary chemistry and expertise for a successful project and can also recommend chemical treatment programs to proactively reduce scale formation (and microbiological fouling) in cooling water systems.
Like all other technologies, due diligence is necessary to determine the feasibility of utilizing methods. Always consult your equipment manuals and guides!

